NCERT Solutions Class 9 Science
The NCERT Solutions in English Language for Class 9 Science Chapter – 4 (Structure of the Atom) has been provided here to help the students in solving the questions from this exercise.
Chapter – 4 (Structure of the Atom)
Questions
1. What are the canal rays?
Answer – Canal rays are positively charged radiations that can pass through perforated cathode plate. These rays consist of positively charged particles known as protons.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer – One proton has one unit positive. Similarly, one electron has one unit negative charge. An atom with one proton and one electron will be electrically neutral. The charges will be balanced inside an atom.
Questions
1. On the basis of Thompson’s model of an atom, explain how the atom is neutral as a whole.
Answer – On the basis of Thomson’s model of an atom, an atom consists of a positively charged sphere. The electrons are embedded in the sphere. The negative and the positive charges are equal in magnitude. Hence, the atom is electrically neutral.
2. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Answer – As per Rutherford’s model of an atom, the positively charged protons are the ones that are present in the atom.
3. Draw a sketch of Bohr’s model of an atom with three shells.
Answer – A sketch of Bohr’s model of an atom with three electron shells is shown below:
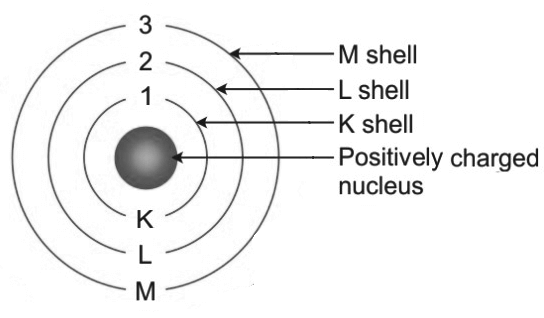
4. What do you think would be the observation if the ∝– particle scattering experiment is carried out using a foil of a metal other than gold?
Answer – In the ∝ – particle scattering experiment, when any other metal foil is used instead of gold, the observation would remain the same. This is because the structure of an atom, when considered individually, remains the same.
Questions
1. Name the three subatomic particles of an atom.
Answer – An atom consists of three subatomic particles:
- Protons – Positively charged
- Electrons – Negatively charged
- Neutrons – Neutral in nature (no charge)
2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer –
Atomic mass of helium atom = 4u, 2 protons in helium nucleus
Atomic mass = number of protons + number of neutrons
4 = 2 + number of neutrons
Number of neutrons = 4 – 2 = 2
Hence, Helium has 2 neutrons.
Questions
1. Write the distribution of electrons in Carbon and Sodium atoms.
Answer – Atomic number of Carbon = 6
Therefore, number of electrons in Carbon = 6
- 2 of them are held in K Shell (as K Shell can hold max 2 electrons)
- 4 of them are held in L Shell (as L Shell can hold max 8 electrons)
So, K Shell – 2 and L Shell – 4
Atomic number of Sodium = 11
Therefore, number of electrons in Sodium = 11
- 2 of them are held in K Shell (as K Shell can hold max 2 electrons)
- 8 of them are held in L Shell (as L Shell can hold max 8 electrons)
Remaining 1 is held in M Shell
So, K Shell – 2, L Shell – 8 and M Shell – 1
2. If the K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer –
K shell can hold 2 electrons.
L shell can hold 8 electrons.
Hence, when both the shells are full, the total number of electrons present in the atom = 2 + 8 = 10 electrons.
Questions
1. How will you find the valency of chlorine, sulphur and magnesium?
Answer –
Chlorine
Atomic number of chlorine = 17
Electronic configuration of chlorine is – 2, 8, 7
Number of valence electrons = 7
Since atom has 7 valence electrons it is easier for the atom to gain 1 electron to achieve an octet.
Valency of atoms gaining 1 electron = 1
Therefore, Valency of chlorine = 1
Sulphur
Atomic number of Sulphur = 16
Electronic configuration of sulphur is – 2, 8, 6
Number of valence electrons = 6
Since the atom has 6 valence electrons it is easier for it to gain 2 electrons to achieve an octet.
Valency of atoms gaining 2 electrons = 2
Therefore, Valency of sulphur = 2
Magnesium
Atomic number of magnesium = 12
Electronic configuration of magnesium is – 2, 8, 2
Number of valence electrons = 2
Since the atom has 2 valence electrons it is easier for it to lose 2 electrons to achieve an octet .
Valency of atoms losing 2 electrons = 2
Therefore, Valency of magnesium = 2
Questions
1. If the number of electrons in an atom is 8 and the number of protons is also 8, then
(i) What is the atomic number of the atom? and
(ii) What is the charge on the atom?
Answer –
Number of electrons = 8
Number of protons = 8
(i) Atomic number is equal to the number of protons in one atom. Since this atom contains 8 orotons, so the atomic is 8.
(ii) This atom contains an equal number of positively charged and negatively charged electrons (8 each), so it has no overall charge. That is, the charge on this atom is 0 (zero).
2. With the help of the given table, find out the mass number of oxygen and sulphur atom.
Table: Composition of Atoms of the First Eighteen Elements with Electron Distribution in Various Shells.
| Name of Element | Symbol | Atomic Number | Number of protons | Number of Neutrons | Number of Electrons | Distribution of Electrons | Valency | |||
| K | L | M | N | |||||||
| Hydrogen | H | 1 | 1 | – | 1 | 1 | – | – | – | 1 |
| Helium | He | 2 | 2 | 2 | 2 | 2 | – | – | – | 0 |
| Lithium | Li | 3 | 3 | 4 | 3 | 2 | 1 | – | – | 1 |
| Beryllium | Be | 4 | 4 | 5 | 4 | 2 | 2 | – | – | 2 |
| Boron | B | 5 | 5 | 6 | 5 | 2 | 3 | – | – | 3 |
| Carbon | C | 6 | 6 | 6 | 6 | 2 | 4 | – | – | 4 |
| Nitrogen | N | 7 | 7 | 7 | 7 | 2 | 5 | – | – | 3 |
| Oxygen | O | 8 | 8 | 8 | 8 | 2 | 6 | – | – | 2 |
| Fluorine | F | 9 | 9 | 10 | 9 | 2 | 7 | – | – | 1 |
| Neon | Ne | 10 | 10 | 10 | 10 | 2 | 8 | – | – | 0 |
| Sodium | Na | 11 | 11 | 12 | 11 | 2 | 8 | 1 | – | 1 |
| Magnesium | Mg | 12 | 12 | 12 | 12 | 2 | 8 | 2 | – | 2 |
| Aluminium | Al | 13 | 13 | 14 | 13 | 2 | 8 | 3 | – | 3 |
| Silicon | Si | 14 | 14 | 14 | 14 | 2 | 8 | 4 | – | 4 |
| Phosphorus | P | 15 | 15 | 16 | 15 | 2 | 8 | 5 | – | 3.5 |
| Sulphur | S | 16 | 16 | 16 | 16 | 2 | 8 | 6 | – | 2 |
| Chlorine | Cl | 17 | 17 | 18 | 17 | 2 | 8 | 7 | – | 1 |
| Argon | Ar | 18 | 18 | 22 | 18 | 2 | 8 | 8 | 0 | |
Answer –
(a) To find the mass number of Oxygen,
Number of protons = 8
Number of neutrons = 8
Atomic number = 8
Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16
Therefore, the mass number of oxygen = 16
(b) To find the mass number of Sulphur,
Number of protons = 16
Number of neutrons = 16
Atomic number = 16
Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
Questions
1. For the symbols H, D and T, tabulate three subatomic particles found in each of them.
Answer – The following table depicts the subatomic particles in Hydrogen (H), Deuterium (D), and Tritium(T).
| Isotope | Symbol | Mass no. | Atomic no. | No. of electrons | No. of protons | No. of neutrons |
| Hydrogen | H | 1 | 1 | 1 | 1 | 0 |
| Deuterium | D | 2 | 1 | 1 | 1 | 1 |
| Tritium | T | 3 | 1 | 1 | 1 | 2 |
2. Write the electronic configuration of any one pair of isotopes and isobar.
Answer –
(a) Isotopes : Isotopes are atoms which have the same number of protons, but the number of neutrons differs. This leads to the variation in mass number too.
Example – Carbon molecule exists as 6C12 and 6C14, but when their electronic configuration is noticed, both have K-2; L-4
(b) Isobars : Isobars are atoms which have the same mass number but differ in atomic number. The electronic configuration of an isobar pair is as follows:
Example – Electronic configuration of 20Ca40 – K-2; L-8; M-8; N- 2
Electronic configuration of 18Ar40 – K-2; L-8; M-8
Exercise
1. Compare the properties of electrons, protons and neutrons.
Answer –
| Property | Electrons | Protons | Neutrons |
| Charge | Negatively charged | Positively charged | No charge. |
| Location | Located outside the nucleus | Located within the nucleus | Located inside the nucleus of an atom |
| Weight | Mass is negligible | 1 a.m.u | 1 a.m.u |
| Affinity | Attracted towards positively charged | Attracted towards negatively charged | Do not get attracted to any charged particle |
2. What are the limitations of J.J.Thomson’s model of the atom?
Answer – Limitations of J.J Thomson’s model of an atom:
- The results of Rutherford’s alpha particle scattering experiment could not be explained by Thomson’s model.
- His concept has a number of significant flaws, one of which being the absence of any experimental data.
- He was unable to explain why atoms are stable.
3. What are the limitations of Rutherford’s model of the atom?
Answer – Limitations of Rutherford’s model of the atom:
- Electrons revolve around the nucleus in a circular path.
- This is not possible because, if a particle follows a circular orbit, it will undergo acceleration (due to change in velocity as a result of continuous change in direction in a circular path).
- During acceleration, charged particles radiate and lose energy.
- After losing all energy, the charged electron will ultimately fall into the nucleus.
- This would make the atom very unstable.
- But we know that atoms are actually stable.
- Hence, there was a flaw in Rutherford’s model of an atom.
4. Describe Bohr’s model of the atom.
Answer – The present concept of atom was given by Neils Bohr. The bohr’s model of atom can be described as follows:
- An atom holds the nucleus at the centre.
- Negatively charged electrons revolve around the nucleus.
- The atoms in it contain distinct orbits of electrons.
- Electrons do not radiate energy when they are in their orbits.
- The distinct orbits are named K, L, M, and N orbits. Numbers used to denote them are n=1, 2, 3, 4

5. Compare all the proposed models of an atom given in this chapter.
Answer –
| Thomson | Rutherford | Bohr |
| Sphere is positively charged. | The nucleus is at the centre and is positively charged, holding the entire mass. | Nucleus is present at the centre and is positively charged |
| Electrons are negatively charged and scattered all through the inside of the sphere. | Electrons are negatively charged, revolving in a well-defined path | Electrons are negatively charged, revolving around but do not radiate energy. |
| Positively charged = negatively charged |
In comparison with the nucleus, the size of the atom is very large. | The distinct orbits are labelled as K, L, M, and N |
| The net charge in the atom is zero. |
Force of attraction of the electrons towards the nucleus is balanced by centrifugal force acting away from it. As a result, electrons are not drawn close to the nucleus. |
6. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer – The rules for writing of distribution of electrons in various shells for the first eighteen elements are:
1. The maximum number of electrons present in a shell is formula—2n2
n = orbit number i.e., 1, 2, 3
Maximum number of electrons in different shells are:
- K shell n = 1 2n2 ⇒ 2(1)2 = 2
- L shell n = 2 2n2 ⇒ 2(2)2 = 8
- M shell n = 3 2n2 ⇒ 2(3)2 = 18
- N shell n = 4 2n2 ⇒ 2(4)2 = 32
7. Define valency by taking examples of silicon and oxygen.
Answer – Valency is the combining capacity of an atom.
Atomic number of oxygen = 8
Atomic number of silicon = 14
| K | L | M | |
| Electronic configuration of oxygen | 2 | 6 | – |
| Electronic configuration of silicon | 2 | 8 | 4 |
Hence, the combining capacity of oxygen is 2 and of silicon is 4.
i.e., Valency of oxygen = 2
Valency of silicon = 4
8. Explain with examples:
(i) Atomic number
(ii) Mass number,
(iii) Isotopes and
(iv) Isobars.
Give any two uses of isotopes.
Answer –
(i) Atomic number – The atomic number of an element is equal to the number of protons in the nucleus of its atom. e.g., Oxygen has 6 protons hence atomic no. = 6.
(ii) Maas number – The total number of protons and neutrons present in one atom of an element is known as its mass number. For example, one atom of sodium element contains 11 protons and 12 neutrons. Hence, the mass number of sodium is 11 + 12 = 23. Similarly, a normal carbon atom has 6 protons and 6 neutrons, so the mass number of carbon is 6 + 6 = 12.
(iii) Isotopes – Atoms of same element with same atomic number but different mass number are called isotopes of that element. For example, Hydrogen has three isotopes, protium (H11), deuterium (H21 or D21) and tritium (H31 or T31). Many elements consist of a mixture of isotopes. Each isotope of an element is a pure substance. The chemical properties of isotopes are similar but their physical properties are different.
(iv) Isobars – Atoms of different elements which have the same mass number but have different atomic numbers are called isobars. For example, calcium, atomic number-20 and argon, atomic number 18 are isobars. Both these elements have same mass number which is equal to 40. Therefore, the total number of nucleons is the same in both the elements.
Uses of Isotopes: Isotopes of some elements have special properties makes them useful in various fields. For example,
1. An isotope of uranium is used as a fuel in nuclear reactors.
2. An isotope of cobalt is used in the treatment of cancer.
3. An isotope of iodine is used in the treatment of goitre.
9. Na+ has completely filled K and L shells. Explain.
Answer – A sodium ion Na+, has 10 electrons in it. Now, the maximum capacity of K shell is 2 electrons and that of L shell is 8 electrons. Taken together, the maximum capacity of K and L shells is 2 + 8 = 10 electrons. A sodium ion Na+ has completely filled K and L shells because its 10 electrons can completely fill up K and L shells.
10. If bromine is available in the form of, say, two isotopes 35Br79 (49.7%) 35Br81 (5,3%) calculate the average atomic mass of bromine atom.
Answer –
The mass of 35Br79 isotope is 79 u and its abundance is 49.7%.
The mass of 35Br81 isotope is 81 u and its abundance is 50.3%.
So, Average atomic mass of bromine = 79 ×
=
= 39.263 + 40.743
= 80.006
= 80u
Thus, the average atomic mass of bromine is 80 u.
11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 8X16 and 8X18 in the sample.
Answer – In order to solve this problem, we will have to suppose that the percentage of one of the isotopes in the sample is x, so that the percentage of the other isotope in the sample will be (100 – x). Now:
The mass of 8X16 isotope is 16u. Suppose its percentage in the sample is x%.
The mass of 8X18 isotope is 18 u. Its percentage in the sample will be (100 – x)%.
So, Average atomic mass of X = 16 ×
But the average atomic mass of X has been given to be 16.2 u. Therefore,
16.2 = 16 ×
16.2 =
16.2 × 100 = 1800 – 2X
2X = 1800 – 1620
2X = 180
X = 180/2
X = 90
Thus, the percentage of the isotope 8X16 in the sample is 90%. The percentage of the isotope 8X18 in the sample will be 100 – 90 = 10%.
12. If Z = 3, what would be the valency of the element? Also name the element.
Answer – By Z = 3, we mean that the atomic number of the element is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron).
Therefore, the element with Z = 3 is lithium (Li).
13. Composition of the nuclei of two atomic species X and Y is given as under:
| X | Y | ||
| Protons | = | 6 | 6 |
| Neutrons | = | 6 | 8 |
Give the mass numbers of X and Y. What is the relation between the two species?
Answer – Mass number = No. of protons + No. of neutrons
So, Mass number of X = 6 + 6 = 12
Mass number of Y = 6 + 8 = 14
Thus, the mass number of X is 12 and that of Y is 14
14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer –
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. (False)
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. (False)
(c) The mass of an electron is about 1/2000 times that of proton. (True)
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. (False)
15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of:
(a) Atomic nucleuse
(b) Electrone
(c) Protone
(d) Neutron
Answer – (a) Atomic nucleus
16. Isotopes of an element have :
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers
Answer – (c) different number of neutrons.
17. Number of valence electrons in Cl- ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer – (b) 8
18. Which of the following is a correct electronic configuration of sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8,1
Answer – (d) 2, 8, 1
19. Complete the following table:
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the Atomic Species |
| 9 | – | 10 | – | – | – |
| 10 | 32 | – | – | – | Sulphure |
| – | 24 | – | 12 | – | – |
| – | 2 | – | 1 | – | – |
| – | 1 | 0 | 1 | 0 | – |
Answer – The following table depicts the missing data:
Atomic number (Z) = Number of protons
Mass number = Number of neutrons + atomic number
(or)
Mass number (A) = Number of neutrons + number of neutrons
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the Atomic Species |
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | Sulphure |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 1 | 0 | 1 | 1 | Protium |

Leave a Reply