NCERT Solutions Class 8 Science
The NCERT Solutions in English Language for Class 8 Science Chapter – 6 (Combustion and Flame) has been provided here to help the students in solving the questions from this exercise.
Chapter – 6 (Combustion and Flame)
1. List conditions under which combustion can take place.
Answer – The conditions under which combustion takes place are as follows:
- Presence of combustible substance.
- Presence of supporter of combustion – oxygen.
- Attainment of ignition temperature.
2. Fill in the blanks.
(a) Burning of wood and coal causes __________of air.
(b) A liquid fuel, used in homes is__________ .
(c) Fuel must be heated to its ____________ before it starts burning.
(d) The fire produced by oil cannot be controlled by___________ .
Answer –
(a) Burning of wood and coal causes pollution of air.
(b) A liquid fuel, used in homes is kerosene.
(c) Fuel must be heated to its ignition temperature before it starts burning.
(d) The fire produced by oil cannot be controlled by water.
3. Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer – CNG plays an important role in reducing pollution among automobiles for the following reasons:
-
It produces less carbon monoxide gas.
-
It produces less carbon dioxide gas.
-
It produces less amount of sulphur dioxide and nitrogen dioxide which cause acid rain.
-
No residue remains after combustion.
Answer –
| LPG | Wood |
|---|---|
| LPG is a gaseous fuel. | Wood is a solid fuel. |
| LPG has a higher calorific value of 55000 kJ/kg. | Wood has a lower calorific value of 17000 to 22000 kJ/kg. |
| On burning,it leaves no residue. | On burning, it leaves ash as residue. |
| It causes less pollution. | It causes more pollution. |
| It is easy to store. | It requires a lot of space. |
| It can be easily transported in cylinders or pipelines. | It is difficult to transport wood. |
| It does not cause any pollution. | It causes pollution due to the residue and smoke. |
5. Give reasons.
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answer –
(a) Water is a good conductor of electricity. If it is used for controlling a fire involving electrical equipments, then the person dousing the fire might get an electric shock. Also, water can damage electrical equipments.
(b) LPG is a better domestic fuel as it does not produce smoke and unburnt carbon particles, which cause respiratory problems.
(c) A piece of paper wrapped around aluminium pipe does not catch fire easily. This is because aluminium, being a metal, is a good conductor of heat. Therefore, heat is transferred from the paper to the metal and the paper does not attain its ignition temperature.
6. Make a labelled diagram of a candle flame.
Answer –
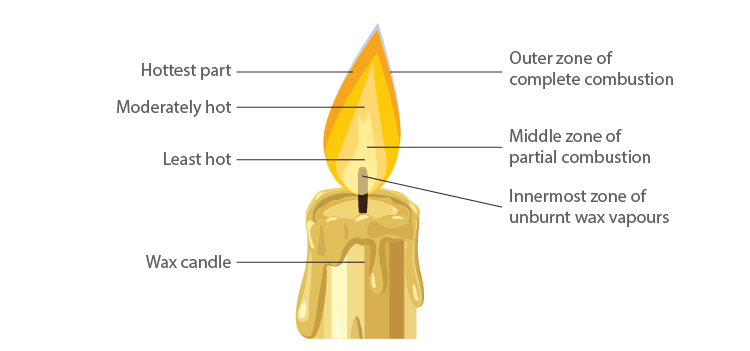
7. Name the unit in which the calorific value of a fuel is expressed.
Answer – The calorific value of a fuel is expressed in kilojoules per kilogram (kJ/kg).
8. Explain how CO2 is able to control fires.
Answer – CO2 is a non-combustible gas and extinguishes fire in two ways:
(i) It is heavier than oxygen and covers the fire like a blanket and cuts off the contact between oxygen and fuel.
(ii) In cylinders, CO2 is kept in the liquid form. When released, it expands enormously. This brings down the temperature of the fuel, which helps in controlling the fire.
9. It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer – Green leaves have a lot of moisture in them. This moisture does not allow them to catch fire easily. However, dry leaves have no moisture in them. Therefore, they catch fire easily.
10. Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer – Goldsmiths use the outermost part/zone of the flame to melt gold and silver. This is because the outermost zone of the flame undergoes complete combustion and is the hottest part of the flame.
11. In an experiment, 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Answer – Heat produced by 4.5 kg of fuel = 180000 kJ
Therefore, heat produced by 1 kg of fuel = kJ/kg = 40,000 kJ/kg
Hence, the calorific value of the fuel if 40,000 kJ/kg.
12. Can the process of rusting be called combustion? Discuss.
Answer – No, because rusting is an exothermic process as heat is liberated during rusting. On the other hand, combustion is a chemical process in which a substance reacts with oxygen to release energy in the form of heat or light.
13. Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Answer – The water in the Ramesh’s beaker will heat up in a shorter time. This is because the outermost zone of a flame is the hottest zone, while the yellow zone (in which Abida had kept the beaker) is less hot.

Leave a Reply